

 海博微信公众号
海博微信公众号
 海博天猫旗舰店
海博天猫旗舰店


 海博微信公众号
海博微信公众号
 海博天猫旗舰店
海博天猫旗舰店




USP42第二增补版新增了章节,增加了一个特定微生物的测试。现将中英文推送如下。
INTRODUCTION 介绍
The tests described in this chapter will allow determination of the absence of Burkholderia cepacia complex(Bcc),which can be detected under the conditions described.
本章节所描述的测试是允许确定BCC 的存在,可在描述的情况下检测。
The tests are designed to determine whether a substance or preparation complies with an established specification for microbiology quality and/or to evaluate whether products ----especially those for inhalation use or aqueous preparations for oral,oromucosal,cutaneous,or nasal use----contain members of the Bcc.
该测试旨在确定一种物质或制剂是否符合已建立的微生物质量标准,并/或评估产品——特别是用于吸入或口服、口服粘膜、皮肤或鼻腔使用的含水制剂——是否含有BCC。
GROEITH-PROMOTING AND INHIBITORY PROPERTIES OF THE MEDIA AND
SUITABILITY OF TESTS FOR ABSENCE OF Bcc
培养基促生长和抑制作用以及BCC 方法适用性
Test each batch of ready-prepared medium and each batch of medium prepared from either dehydrated medium or ingredients.
测试每一批预制的培养基,以及每一批由脱水培养基或原料制成的培养基。
Preparation of Test Strains 测试菌株的准备
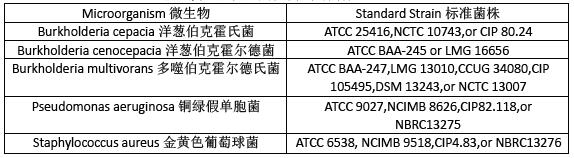
Use standardized stable suspensions of test strains(see Table1)NMT 5 passages removed from the original strain culture.
使用标准的稳定的测试用菌悬液,从原始菌株不超过5 代。
Table1.Test Strains of Microorganisms for Growth Promotion and Suitability Testing
表1 促生长及适用性测试的菌株
Microorganism 微生物
Grow each of the test strains separately in Soy-Casein Digest Broth or on Soybean-Casein Digest Agar at 30℃-35℃ for 18-24h.
分别在TSB/TSA 中培养上述每一个菌株于30℃-35℃ ,18-24h.
Use Buffered sodium Chloride-peptone Solution pH7.0 or Phosphate Buffer Solution pH7.2 to make the test suspensions.Use the suspensions within 24h if stored at 2℃-8℃.If purchased,follow the supplier’s introductions. If self-prepared cultures are used,follow a validated procedure (such as in Microbial Enumeration Test)for preparation. Use a challenge inoculum of NMT 100 colony-forming units(cfu) for growth promotion and suitability testing.
使用pH7.0 氯化钠蛋白胨缓冲液或pH7.2 磷酸盐缓冲液制备菌悬液。若保存在2o-8o 在24 小时内使用菌悬液。若是采购,按照供应商的说明书。如果使用自制培养物,请遵循经过验证的程序(如微生物计数试验)进行制备。使用不超过100cfu的挑战菌进行促生长试验和适用性试验。
NEGATIVE CONTROLS 阴性对照
Include a negative control to verify the testing conditions. There must be no growth of microorganisms. A negative control is also performed when testing the products as
described in Testing of Products.
为确认测试条件需包含阴性对照。必须无微生物生长。在产品测试中描述的在产品测试过程中也需要进行阴性对照。
TEST FOR GROWTH-PROMOTING PROPERTIES, SOLID MEDlA
固体培养基的促生长试验
Perform the Surface-Spread Method (see Microbial Enumeration Test, Growth Promotion Test, Suitability of the Counting Method and Negative Controls, Suitability of the Counting Method in the Presence of Product, Recovery of Microorganisms in the Presence of Product, Plate-Count Methods), inoculating each plate with a small number(NMT 100 cfu) of the appropriate microorganism (see Table 2). Incubate at the special temperature for NMT the shortest period of time specified in the test. Growth of the microorganism comparable to that previously obtained with a previously tested and approved batch of medium occurs.
采用涂布法(见微生物计数法,促生长试验,计数方法适用性和阴性对照,产品存在时的计数方法适用性,产品存在时的微生物回收率,平皿法),接种不超过100cfu 的合适的微生物(见表2)。在特定的温度下培养不超过特定培养时间的最短时间。微生物的生长可与以前通过先前测试和批准的一批培养基获得的微生物相媲比较。
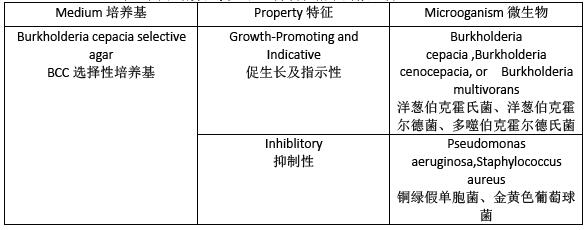
Table2.Microoganisms for the Growth-Promoting,Inhibitory,
and Indicative properties of the Media
表2.用于培养基促生长、抑制和指示的微生物
TEST FOR INHIBITORY PROPERTIES, SOLID MEDIA
固体培养基的抑制性测试
Inoculate the appropriate medium with at least 100 cfu of the appropriate microorganism. Incubate at the specified temperature for NLT the longest period of time specified in the test .Inhibition of growth of the indicated microorganisms occurs(see Table 2).
使用正确的微生物接种至少100cfu 至合适的培养基中。在特定的温度下培养不低于特定培养时间的最长时间。抑制规定的微生物的生长(见表2)。
TEST FOR INDICATIVE PROPERTIES指示性测试
Perform the Surface-Spread Method (see Microbial Enumeration TestsGrowth Promotion Test, Suitability of the Counting Method and Negative Controls, Suitability of the Counting Method in the Presence of Product, Recovery of Microorganisms .In the Presence of Product, Plate-Count Methods), inoculating each plate with a small number(NMT 100 cfu) of the indicated microorganism. Incubate at the specified temperature for a period of time within the range specified in the test. Colonies are comparable in appearance and indication reactions to those previously obtained with a previously tested and approved batch of medium (see Table 2).
采用涂布法(见微生物计数法,促生长试验,计数方法适用性和阴性对照,产品存在时的计数方法适用性,产品存在时的微生物回收率,平皿法),接种不超过100cfu 的合适的微生物(见表2)。在规定的温度下,在试验规定的范围内培养一段时间。菌落在外观和指示反应方面与以前通过先前测试和批准的一批培养基获得的菌落相当。
Suitability of the Test Method 测试方法适用性
The ability of the test to detect Bcc in the presence of the product to be tested must be established. The incubation time for the method suitability should not exceed the shortest incubation period specified. Suitability must be confirmed if there is a change in testing performance or a change in the product that may affect the outcome of the test.
必须建立在待测产品存在的情况下检测BCC 的能力。方法试用性的培养时间不应超过规定的最短培养时间。如果测试性能发生了变化,或者产品发生了可能影响测试结果的变化,则必须确认适用性。
For each new product to be tested, perform the sample preparation as described in Testing of Products. At the time of mixing, add each test strain in the prescribed growth medium. Inoculate the test strains individually. Use a number of microorganisms equivalent to NMT 100 cfu in the inoculated test preparation.
对每一个要测试的新产品,按照产品测试中描述的方法进行样品准备。在混合的时候,在规定的生长培养基中加入每个测试菌株。分别接种试验菌株。在接种试验制剂中使用一些相当于不超过100 cfu 的微生物。
Perform the test as described in Testing of Products, using the shortest incubation period prescribed. Bcc microorganisms must be detected with the indication reactions described in Interpretation.
按照产品测试中描述的方法,使用规定的最短培养时间进行测试。BCC 必须能检测出在解释中描述的指示性反应。
Any antimicrobial activity of the product necessitates a modification of the test procedure (see Microbial Enumeration Test, Growth Promotion Test, Suitability of the Counting Method and Negative Controls, Suitability of the Counting Method in thePresence of Product,
Neutralization/Removal of Antimicrobial Activity).
该产品的任何抗菌活性都需要修改测试程序(见微生物计数法,促生长试验,计数方法的适用性和阴性对照,产品存在时的计数方法适用性,抑菌活性的中和/去除)。
TESTING OF PRODUCTS 产品测试
Sample Preparation and Pre-Incubation 样品制备和预培养
Prepare a sample using a 1-in-10 dilution of NLT 1 g of the product to be examined.Use 10ml or the quantity corresponding to 1 g or 1ml to inoculate a suitable amount(determined as described in Suitability of the Test method) of Soybean-Casein Digest Broth or an appropriate dilution of Soybean-Casein Digest Broth as determined during method suitability (for example ,a 1:10 dilution may be required when conducting optional testing of pharmaceutical waters). Then mix and incubate at 30o-35ofor 48-72h.
用1:10 倍稀释的不少于1g 的待测样品制备供试液。用10ml 或相当于1g 或1ml的合适的(根据测试方法的适用性确定)供试液接种至TSB 或在方法适用性确定的适当体积的TSB 中(例如,在进行制药用水可选检测时,可能需要1:10 稀释)。然后混合及在30℃-35℃ 培养48-72h.
Selection and subculture 选择和继续培养
Subculture by streaking on a plate of Burkholderia cepacia selective agar(BCSA),and
incubate 30o-35o for 48-72h.
在BCSA 上划线继续培养,30o-35o 培养48-72h。
Interpretation 解释
The possible presence of Bcc is indicated by the growth of greenish-brown colonies with yellow halos, or white colonies surrounded by a pink-red zone on BCSA .Any growth on BCSA is confirmed by identification tests. See Microbial Characterization,Identification,and Strain Typingfor additional information.
BCC 可能存在的指示性特征为生长带有黄色光环的绿棕色菌落,或在BCSA 显示被粉红色区域包围的白色菌落。任何在BCSA 上的菌落需要通过鉴定测试确认。更多信息见微生物特性、鉴定和菌株分型.
The product complies with the test if clones of the types described are not present or if the confirmatory identification tests are negative.
如果所述类型的菌落不存在,或者确认性鉴定测试为阴性,则产品符合测试。
RECOMMEND CULTURE MEDIA 推荐的培养基
[Note-This section is given for information.][注:本节内容仅供参考。]The following solutions and culture media have been found satisfactory for the purposes for which they are prescribed in the tests in this Pharmacopeia . Other media may be used provided that their suitability can be demonstrated.
下列溶液和培养基已被发现符合本药典测试规定的目的。如果能够证明其他培养基的适用性,则可以使用其他培养基。
Stock Buffer Solution 储备缓冲液
Transfer 34 g of potassium dihydrogen phosphate to a 1000-ml volumetric flask,dissolve in 500 ml of Purified Water adjust with sodium hydroxide to a pH of 7.2±0.2,add Purified Water to volume,and mix. Dispense in containers,and sterilize. Store at a temperature of 2o-8o.
将34 克磷酸二氢钾转移到1000 毫升的容量瓶中,溶解于500 毫升纯净水中,用氢氧化钠调至pH 值7.2±0.2,加入纯化水,搅拌均匀。分装于容器内,并灭菌。储存温度为2℃-8℃。
Phosphate Butter Solution pH 7.2 pH7.2 磷酸盐缓冲液
Prepare a mixture of purified Water and Stock Buffer Solution (800:1 v/v),and sterilize.
准备纯化水和储备缓冲液(800:1 v/v)混合,灭菌。
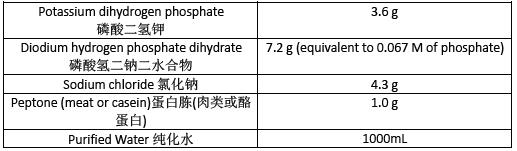
Buffered Sodium Chloride-Peptone Solution pH7.0 pH7.0 氯化钠-蛋白胨缓冲液
Prepare Buffered Sodium Chloride-Peptone Solution pH 7.0 as directed in table 3.Sterilize in an autoclave using a validated cycle.
按表3 所示制备pH 7.0 氯化钠-蛋白胨缓冲液。在灭菌锅中使用经过验证的程序灭菌。
Table 3 表3
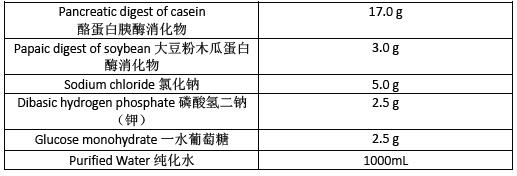
Soybean-Casein Digest Broth 胰酪胨大豆肉汤
Prepare Soybean-Casein Digest Broth as directed in table 4 .Adjust the pH so that after sterilization it is 7.3±0.2 at 25o.Sterilize in an autoclave using a validated cycle.
按表4 制备TSB。调节pH 使得灭菌后在25o 为7.3±0.2.在灭菌锅中使用经过验证的程序灭菌。
Table 4 表4
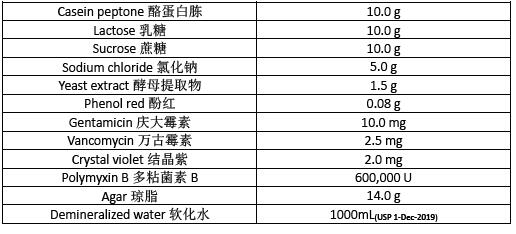
Burkholderia cepacia Selective Agar 洋葱伯克霍尔德菌选择性琼脂
Prepare BCSA as directed in Table 5. When preparing media in-house,first prepare the base ingredients without the antibiotics. Adjust the pH so that after sterilization it is 6.8±0.3 at 25o.Sterilize in an autoclave using a validated cycle.Cool the base medium to 45o-50o and add a 1% solution of the sterile filtered antibiotics ,mix,and pour into the plates.
按表5 制备BCSA。当在实验室准备培养基时,首先准备没有抗生素的基本成分。调节pH 使得灭菌后在25o 为6.8±0.2.在灭菌锅中使用经过验证的程序灭菌。将基础培养基冷却至45℃-50℃,加入1%的无菌过滤抗生素溶液,混合后倒入平板中。
Table 5 表5
上一篇:微生物计数试验——营养和添加剂
下一篇:没有了!
| 相关文章: | ||



